Ethylene Oxide
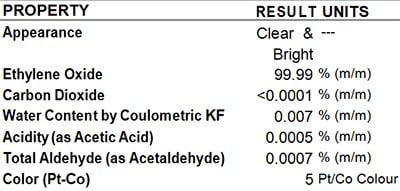
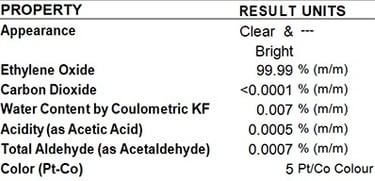
SGS Analysis Report
Pure EO
Distributors usually purchase pure EO in large quantities and then add CO2 to produce a mixture at their own plant. A 20GP (approx. 10 tons of EO) or 40GP (approx. 15 tons) container will effectively spread the transportation and additional costs.
Containers for pure EO can range from 30L seamless steel cylinders to 1000L welded cylinders, and to ensure the quality of EO, aluminum cylinders are recommended below 100L, and stainless steel cylinders are recommended above 100L. The shelf life of EO in both aluminum and stainless steel cylinders can be more than one year.


EO+CO2 MIXTURE
90:10 / 80:20 / 70:30 / 60:40 / 55:45
40:60 / 30:70 /20:80 / 9:91 / or
Customized to user requirements


How to choose a container for EO
★Rust will accelerate the polymerization reaction of EO, so containers made of carbon steel are rarely used for preserving EO in China, and stainless steel or aluminum Cylinders are recommended.
★Pure EO is low pressure and can be stored in seamless or welded cylinders.
★EO+CO2 mixture is high pressure, must use seamless cylinders, the capacity of seamless cylinders are generally less than 100L.
EO steri-gas Cartridges
Product Details of EO Cartridge
Minimum Order Quantity
100 Piece
Purity
Usage/Application
100 % EO Gas
40 /70/100/170/340 gram
Packaging Size
Hospital , Pharma , Medical Device company
The Secrets of Ethylene Oxide
Some assume that the vaporization (boiling) temperature of EO ≈ 10.7 °C, is a “critical danger temperature,” and that exceeding this value immediately leads to uncontrollable risk.
Boiling Point ≠ Transport Hazard Threshold
The boiling point is defined at atmospheric pressure, while risks in closed transport containers depend on the relationship between EO’s vapor pressure and the vessel’s design pressure.Regulations use the criterion “50 °C / 1 MPa” rather than 10.7 °C, based on EO’s physical property curves and container design standards ([NIST WebBook]
Transport regulations (UN/ADR/IMDG) explicitly describe EO (or EO with nitrogen) as “… up to a total pressure of 1 MPa (10 bar) at 50 °C.” This means that, from both engineering and regulatory perspectives, EO may be safely loaded and transported under conditions of 50 °C and ≤ 1 MPa total pressure. The regulatory limits already account for the increase of vapor pressure with temperature, together with safety provisions such as tank design, relief devices, and inspection requirements ([UNECE]
Technical Analysis:
Vapor Pressure and Temperature
The normal boiling point of EO is approximately 10.7 °C. However, the boiling point only indicates the temperature at which a pure liquid transitions completely to vapor at 1 atm; in a closed container, a liquid–vapor equilibrium is established, and the pressure is governed by the saturated vapor pressure.
Using authoritative property data (Antoine parameters from the NIST WebBook), the saturated vapor pressure of EO can be precisely calculated at any temperature. The results show that below 50 °C, EO’s vapor pressure increases with temperature but remains in the range of roughly 1–10 bar (depending on the specific temperature point), rather than exhibiting an “instantaneous, infinite rise.” The regulatory limit of “up to 50 °C and a total pressure ≤ 1 MPa” is directly based on these physical property correlations together with container design capacity. (Antoine coefficients and data available in NIST WebBook.) ([NIST WebBook]



